My Blog
Tips, Resources, and Strategies for High School Science

How to Solve Limiting Reactants Problems
Limiting reactants problems are a common type of problem in chemistry that many students struggle with. The limiting reactant is the one that is used up first, leaving the other reactants in excess. In these types of problems, you are given the amounts of two or more reactants and asked to determine which one is the limiting reactant. And sometimes, how much product can be produced. However, with the right approach, these problems can be easily solved. Here’s a step-by-step guide on how to solve limiting reactants problems in chemistry using an example question:
Question: What is the limiting reactant if 28 g of N2 reacts with 25 g of H2 to form NH3?
The reaction between nitrogen and hydrogen to form ammonia is a well-known example of a limiting reactant problem. The balanced chemical equation for this reaction is:
N2 + 3H2 → 2NH3
To solve this problem, we need to determine which reactant is the limiting reactant. In this example, we are given the mass of each reactant. This means we need to use stoichiometry to determine which reactant limits the amount of product formed.
STEP 1: CONVERT THE MASSES OF EACH REACTANT TO MOLES
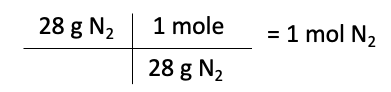
The first step is to convert the masses of each reactant to moles. To do this, we use the molar mass of each element or compound, which can be found in the periodic table. The molar mass of nitrogen is 28 g/mol, while the molar mass of hydrogen is 2 g/mol.
Moles of N2
Moles of H2
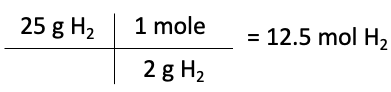
STEP 2: USE THE BALANCED EQUATION TO DETERMINE THE MOLES OF PRODUCT FORMED
Next, we need to use the balanced equation to determine the number of moles of product that can be formed from each reactant, known as the mole ratio. For this equation, the ratio of moles of nitrogen to ammonia is 1:2. Also, the ratio of moles of hydrogen to ammonia is 3:2.
Moles of NH3 formed from N2
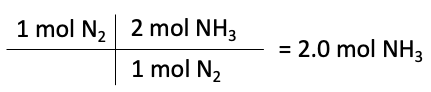
Moles of NH3 formed from H2
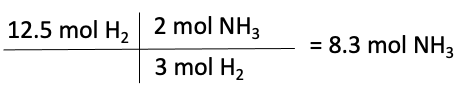
STEP 3: DETERMINE THE LIMITING REACTANT
The limiting reactant is the reactant that produces the least amount of product. The calculations above show that nitrogen produces 2 moles of NH3, while hydrogen produces 8.3 moles of NH3. Therefore, nitrogen is the limiting reactant in this reaction, and hydrogen is the reactant in excess.
STEP 4: CALCULATE THE MAXIMUM AMOUNT OF PRODUCT FORMED
Finally, we can use the moles of the limiting reactant to determine the maximum amount of product that can be formed. In this case, nitrogen produces 2 moles of NH3. So, using the grams of N2 to calculate the maximum amount, in grams, of NH3 that can be formed is:
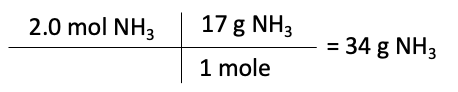
Therefore, the maximum amount of NH3 that can be formed in this reaction is 34 grams.
Watch this video with an example of limiting reactants and more practice problems!
All Rights Reserved 2023 - (C) TheScienceMentor.com -TM | Terms & Conditions | Privacy Policy | Disclaimers
